Almost a year ago, on my old blog, I brought up the issue of the Suess effect. Go read that post if you don’t remember what the Suess effect is. If you want an executive summary, digest this: The burning of low-14C fossil fuels (because the fuels are old and the 14C has all decayed), lowers the total atmospheric ratio of 14C relative to other isotopes of carbon. The carbon in the atmosphere has become enriched in the lighter isotopes of carbon, 13C and 12C, as low-14C coal, oil, and natural gas get oxidized.
However, this same principle applies to 13C vs. 12C: plants preferentially take up 12C over 13C; lighter isotopic ratios are typical of photosynthesis-filtered carbon populations. So when isotopically light fossil fuels are burned, once again, the overall isotopic ratio of the atmosphere becomes “seasoned” with lightweight CO2. The measure of the ratio of 13C to 12C is usually expressed as a value called δ13C. Higher δ13C = more 13C than 12C. Lower δ13C = more 12C than 13C. Here’s data from Mauna Loa (Hawaii) and the South Pole (Antarctica), showing variations in atmospheric δ13C over the past ~30 years:
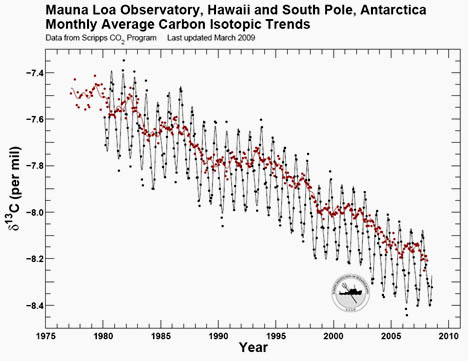 Image: Ralph Keeling’s lab at Scripps
Image: Ralph Keeling’s lab at Scripps
Here, you can see the annual seasonal cycle of the Earth’s plants “breathing” in and out (northern hemisphere photosynthesis dominates this signal), with higher δ13C values each boreal summer, as modern plants preferentially suck the 12C out of the air. The values decrease each winter as photosynthesis reaches its minimum, and decay pumps some of that light carbon (enriched in 12C) back into the air. And, of course, overall the trend is negative, showing the increasing abundance of fossil fuel carbon in our planet’s atmosphere.
A new paper by Peter Swart and colleagues in Geophsyical Research Letters takes a look at scleractinian corals, to see what they’re recording about the changing composition of our atmosphere. Corals are marine organisms, but the atmosphere and the oceans trade many constituents back and forth, including carbon dioxide.
The team collected corals from Florida and elsewhere in the Caribbean, and assessed their carbon isotopes over time. They compared these numbers with 27 other published studies of coral isotopic composition data. They found that 64% of the total corals studied (in the entire world ocean) show a statistically significant trend towards lower isotopic values (lower δ13C) over the period from 1900 to 1990. The average rate of decline is -0.01‰ per year. In the Atlantic Ocean, though, there was a higher rate of change in the isotope ratios, about -0.019‰ per year for the period of 1960 to 1990, which is identical to the rate of decrease of δ13C in the atmosphere over that same span of time. They note that the Atlantic has a higher “inventory” of anthropogenically-liberated CO2 than the Pacific or Indian Oceans. (I’m not sure I understand why this should be the case; chime in if you can explain it to me.)
Please take a look at Figure 2 from the new paper:
 Image: Swart, et al. (2010), Figure 2
Image: Swart, et al. (2010), Figure 2
In this graph, δ13C is plotted on the vertical axis, for both the atmosphere (data from Ralph Keeling) and from the sampled corals (and some published values sponges, too). Time (1800 to 2000) is plotted on the horizontal axis. Atlantic values are green. Pacific and Indian values are Barney-purple. The cited sclerosponges sampled (from the Atlantic) are a deep purple color. The atmospheric δ13C measurements are in light blue. You can see that over time, all of the values show a decrease in δ13C. The Atlantic signal stays truest to the atmospheric signal, while the Indian and Pacific signals show greater variability.
Take home point: whether we’re sampling the atmosphere directly or whether we are reading atmospheric isotopic changes filtered through the oceans via animal skeletons, we’re getting the same story: this study found that corals are reliable proxies for atmospheric changes. The burning of fossil fuels is liberating low-isotopic-weight carbon from subterranean reservoirs. This contributed carbon is fluffing up the overall isotopic weight of the carbon in our atmosphere. The atmosphere is communicating this change to us in an isotopic tune. Corals are harmonizing with the atmosphere, singing the same song at the same time.
____________________________________________
Peter K. Swart, Lisa Greer, Brad E. Rosenheim, Chris S. Moses, Amanda J. Waite, A. Winter, Richard E. Dodge, & Kevin Helmle (2010). The 13C Suess effect in scleractinian corals mirror changes in the anthropogenic CO2 inventory of the surface oceans GEOPHYSICAL RESEARCH LETTERS, : https://doi.org/10.1029/2009GL041397.
Major hat tip to M.J. Viñas of AGU, who shared a copy of the article with me. Thanks!

sweet! I wonder if someone can take these figures of declining carbon ratios and calculate how much of the anthropogenic emissions are being buffered by the ocean..I know there are numbers already available but maybe this is another way to estimate the atmosphere ocean carbon dioxide exchange.